
Spontaneous intestinal perforation among very preterm infants in China: a multicenter cohort study
Highlight box
Key findings
• Around 1% of very preterm infants (VPIs) in China developed spontaneous intestinal perforation (SIP), associated with an alarmingly high risk of mortality and morbidity. Over 90% of VPIs with SIP underwent laparotomy as initial or subsequent surgical treatment.
What is known and what is new?
• Spontaneous intestinal perforation has become a dominant surgical bowel condition affecting preterm infants, but there are currently no data on the burden of SIP in Chinese neonatal intensive care units (NICUs).
• This study provides a comprehensive description of the prevalence, management, and prognosis of spontaneous intestinal perforation in very preterm infants across China.
What is the implication, and what should change now?
• Effective and evidence-based strategies are needed for the prevention and management of SIP.
Introduction
Spontaneous intestinal perforation (SIP) is one of the most serious gastrointestinal complications of preterm infants. SIP is characterized by isolated intestinal perforation with relatively normal surrounding tissue, usually occurring at the antimesenteric border of the terminal ileum in the first week of life (1,2). The incidence of SIP is 1–2% among infants born <1,500 g or <32 weeks’ gestation (3-5), and is as high as 3–8% among extremely low birth weight (ELBW) infants (6,7). In recent years, significant efforts have been made to prevent preterm infants from necrotizing enterocolitis (NEC) with decreasing incidence. In contrast, the incidence of SIP has either remained steady or even increased (8). SIP has gradually become a dominant surgical bowel condition affecting the most vulnerable infants (8). SIP significantly increases the mortality and morbidities of preterm infants. The mortality rate is 29–53% in ELBW infants with SIP, approximately twice that in those without SIP (7,9-11). Major morbidities, such as bronchopulmonary dysplasia (BPD) (45–77%), severe retinopathy of prematurity (ROP) (26–28%), and neurodevelopmental impairment at 18–22 months corrected age (63–67%), are also more common in SIP survivors (4,9,11-13). Consequently, SIP is drawing increasing attention. To better understand the epidemiology, treatment practices, and outcomes of SIP, several large multicenter studies have been conducted around the world (4,10,14-16).
During the past two decades, more and more very preterm infants [VPIs, <32 weeks’ gestational age (GA)] have been treated and survived in China, while there are currently no data on the burden of SIP in Chinese neonatal intensive care units (NICUs). Therefore, the present study used the largest cohort of VPIs in China from the Chinese Neonatal Network (CHNN), aiming to provide a comprehensive description of the incidence, management, and outcomes of SIP among VPIs admitted to Chinese NICUs. This article is written following the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-584/rc).
Methods
Study setting and population
This study is a retrospective cohort study using the CHNN database. The CHNN is a collaborative research and quality improvement network of tertiary NICUs in China (17,18). There were 57 hospitals in 2019 and 70 hospitals in 2020 enrolled in CHNN, covering major tertiary perinatal centers and free-standing children’s hospitals from 25 provinces across China. A standardized neonatal database has been established by CHNN to monitor outcomes and clinical practices of all preterm infants born with GA <32 weeks or birth weight <1,500 g and admitted to participating hospitals (19).
The current study included all infants of 24+0–31+6 weeks’ gestation admitted to NICUs participating in CHNN within seven days after birth between January 1, 2019, and December 31, 2020. Infants with congenital gastrointestinal malformations, NEC stage ≥2 based on modified Bell’s staging criteria (20), and missing information on intestinal perforation were excluded.
Data collection
Patient data from medical records were collected and entered electronically into a customized program with built-in error checking by trained abstractors of each site. Operations and definitions of each item in the program were elucidated by a standard manual. Patient identities were kept confidential when retrieved for analysis (19). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Board of Children’s Hospital of Fudan University (No. #CHFU 2018-296) and recognized by all participating hospitals. A waiver of informed consent was granted owing to the use of deidentified patient data.
Exposure
Eligible infants were divided into two groups: infants with and without SIP. SIP was diagnosed based on radiological findings of intestinal perforation and absence of radiological features of intestinal ischemia (fixed dilated bowel loops, pneumatosis intestinalis, and so on), or intraoperative surgical report indicating SIP (4).
Outcomes
The primary outcome was survival without any of the following major morbidities: late-onset sepsis (LOS), BPD, periventricular leukomalacia (PVL), and severe ROP. The secondary outcomes included overall death, any of the major morbidities, growth assessment at discharge [growth velocity and extrauterine growth restriction (EUGR)], and treatment during hospitalization (central venous catheter, invasive ventilation, parenteral nutrition, blood transfusion, and length of NICU stay).
Definitions
In the CHNN cohort, some infants were discharged against medical advice (their caregivers chose to terminate treatment and have them leave the hospital before the attending physicians recommended discharge) during hospitalization. In this study, infants who were discharged against medical advice within two weeks after the onset of SIP were considered to have incomplete care for SIP. If the infants who were discharged against medical advice still required intensive care (invasive or noninvasive mechanical ventilation, inotrope infusion, or total parenteral nutrition) at the time of discharge, they were assumed to die after discharge (21). Overall death included in-hospital death and death after discharge. SIP-related death referred to death that occurred within two weeks of SIP onset or the cause of death was recorded as SIP in the medical record. Antibiotics prescribed from the day of SIP diagnosis were considered as medical treatment for SIP.
GA was determined in a hierarchical order based on the date of in vitro fertilization, prenatal ultrasound, last menstrual period, and obstetric and pediatric estimate. Small for GA (SGA) was defined as birth weight <10th percentile for GA and sex according to Fenton 2013 growth charts (22). Intensive resuscitation at the delivery room included chest compression >30 seconds and the use of epinephrine. Respiratory distress syndrome was diagnosed according to clinical signs, radiologic features, and/or treatment with surfactant replacement. Intraventricular hemorrhage (IVH) was classified according to Papile’s criteria and grade ≥3 was considered severe (23). Early-onset sepsis was defined as a positive bacterial culture from blood or cerebrospinal fluid before seven days of age and LOS was after seven days of age. BPD was defined as oxygen dependency at 36 weeks postmenstrual age or at discharge whichever comes first (24). PVL was defined as periventricular cysts presented on brain magnetic resonance imaging or ultrasound. Severe ROP was defined as stage ≥3 retinopathy according to the International Classification (25). Growth velocity was calculated as the average grams gained per kilogram of weight per day between birth and discharge. EUGR was diagnosed according to: (I) discharge weight <10th percentile; (II) z-score change between birth and discharge weight >2. Fenton 2013 curves were used before 50 weeks postmenstrual age and the World Health Organization growth charts thereafter (26).
Duration of fasting and antibiotics for SIP, time to first full enteral feeding, rates of discharge with enterostomy and major morbidities, growth assessment, times of transfusion, length of NICU stay, and duration of central venous catheter, invasive ventilation, and parenteral nutrition during hospitalization were calculated among SIP survivors.
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 23 (RRID: SCR_016479). Median and interquartile range (IQR) were used to describe continuous variables, whereas frequencies and percentages were used to describe categorical variables. Continuous variables were compared using the Mann-Whitney U test and the Kruskal-Wallis H test. Categorical variables were compared using Chi-squared or Fisher’s exact test. Trend tests were performed using linear-by-linear association. Logistic regression models were used to determine the association between SIP and neonatal outcomes, controlling for GA, SGA, sex, Apgar score <7 at 5 min, and antenatal steroids. The covariates were selected based on previous evidence (3,4). A two-tailed P<0.05 was considered significant.
Results
Incidence and case fatality rate of SIP
Among 15,814 eligible infants, 150 (1.0%) developed SIP (Figure 1). The median age of SIP onset was four (IQR 2–6) days (Table 1). Infants with lower GA had a higher incidence of SIP (P=0.02).

Table 1
| Incidence, case fatality rate and medical treatment | Gestational age | Total | P value | |||
|---|---|---|---|---|---|---|
| 24+0–25+6 weeks | 26+0–27+6 weeks | 28+0–29+6 weeks | 30+0–31+6 weeks | |||
| Number of infants | 532 | 2,005 | 5,269 | 8,008 | 15,814 | |
| Incidence of SIP, n/N (%) | 13/532 (2.4) | 22/2,005 (1.1) | 44/5,269 (0.8) | 71/8,008 (0.9) | 150/15,814 (1.0) | <0.01 |
| Age of SIP onset, days, median (IQR) | 7 (6, 9) | 6 (4, 9) | 4 (2, 5) | 3 (2, 5) | 4 (2, 6) | <0.01 |
| Case fatality rate of SIP†, n/N (%) | ||||||
| Among all SIP infants | ||||||
| Overall death | 6/13 (46.2) | 7/20 (35.0) | 13/42 (31.0) | 15/65 (23.1) | 41/140 (29.3) | 0.32 |
| SIP-related death | 4/13 (30.8) | 7/20 (35.0) | 12/42 (28.6) | 15/65 (23.1) | 38/140 (27.1) | 0.70 |
| Among SIP infants receiving complete care | ||||||
| Overall death | 3/10 (30.0) | 1/14 (7.1) | 5/34 (14.7) | 3/53 (5.7) | 12/111 (10.8) | 0.09 |
| SIP-related death | 1/10 (10.0) | 1/14 (7.1) | 4/34 (11.8) | 3/53 (5.7) | 9/111 (8.1) | 0.72 |
| Medical treatment for SIP‡, median [IQR] | ||||||
| Duration of fasting for SIP, days | 10 [9, 12] | 8 [5, 13] | 10 [8, 13] | 11 [9, 14] | 10 [8, 13] | 0.27 |
| Time to first full enteral feeding after SIP, days | 54 [33, 102] | 41 [26, 57] | 35 [27, 49] | 29 [23, 40] | 32 [25, 45] | 0.03 |
| Duration of antibiotics for SIP, days | 24 [11, 25] | 7 [5, 15] | 11 [7, 19] | 10 [8, 15] | 11 [7, 16] | 0.12 |
†, ten SIP infants were lost to follow-up with unknown survival status, so they were not included in the calculation of case fatality rate. ‡, calculated among SIP survivors. SIP, spontaneous intestinal perforation; VPIs, very preterm infants; IQR, interquartile range.
Ten (6.7%) SIP infants were lost to follow-up due to transfer to non-CHNN hospitals with unknown survival status, so the case fatality rates were calculated among the remaining 140 infants. Overall, a total of 41 (29.3%) infants with SIP died during hospitalization, and 38 (27.1%) cases were identified as SIP-related deaths (Table 1). There were 38 (27.1%) infants discharged against medical advice, among which 29 cases occurred within two weeks of SIP onset and were considered not to receive complete care for SIP. Among 111 infants with complete care, twelve (10.8%) infants died during hospitalization, and nine (8.1%) were SIP-related deaths (Table 1). There was no significant difference in overall or SIP-related case fatality rates among different GA groups, either in all infants or in infants with complete care.
Medical treatment for SIP
The median time to reach first full enteral feeding after SIP was 32 (IQR 25–45) days, with the median duration of fasting of 10 (IQR 8–13) days (Table 1). Infants with lower GA showed an even longer time to reach full enteral nutrition after SIP. The median course of antibiotics prescribed for SIP was 11 (IQR 7–16) days and similar among different GA groups.
Surgical management for SIP
Of all infants with SIP, 28 (18.7%) did not receive any surgical intervention (excluding needle aspiration of free intraperitoneal air), of which two were transferred out and lost to follow-up, eighteen were discharged against medical advice, and the remaining eight received complete care. Among the 26 non-surgical infants with known survival status, 22 (84.6%) infants died, all SIP-related (Table 2).
Table 2
| Case fatality rate and medical treatment | No surgical management | Any surgical management | |||
|---|---|---|---|---|---|
| Total | Laparotomy only | Drainage only | Laparotomy after drainage | ||
| Number of infants | 28 | 122 | 84 | 9 | 29 |
| Surgical management for SIP, n/N (%) | 28/150 (18.7) | 122/150 (81.3) | 84/122 (68.9) | 9/122 (7.4) | 29/122 (23.8) |
| 24+0–25+6 weeks | 4/13 (30.8) | 9/13 (69.2) | 3/9 (33.3) | 3/9 (33.3) | 3/9 (33.3) |
| 26+0–27+6 weeks | 6/22 (27.3) | 16/22 (72.7) | 11/16 (68.8) | 3/16 (18.8) | 2/16 (12.5) |
| 28+0–29+6 weeks | 8/44 (18.2) | 36/44 (81.8) | 25/36 (69.4) | 1/36 (2.8) | 10/36 (27.8) |
| 30+0–31+6 weeks | 10/71 (14.1) | 61/71 (85.9) | 45/61 (73.8) | 2/61 (3.3) | 14/61 (23.0) |
| Case fatality rate of SIP†, n/N (%) | |||||
| Among all SIP infants | |||||
| Overall death | 22/26 (84.6) | 19/114 (16.7) | 13/77 (16.9) | 4/8 (50.0) | 2/29 (6.9) |
| SIP-related death | 22/26 (84.6) | 16/114 (14.0) | 11/77 (14.3) | 3/8 (37.5) | 2/29 (6.9) |
| Among SIP infants receiving complete care | |||||
| Overall death | 4/8 (50.0) | 8/103 (7.8) | 5/69 (7.2) | 2/6 (33.3) | 1/28 (3.6) |
| SIP-related death | 4/8 (50.0) | 5/103 (4.9) | 3/69 (4.3) | 1/6 (16.7) | 1/28 (3.6) |
| Medical treatment for SIP‡, median [IQR or range] | |||||
| Duration of fasting for SIP, days | 26 [26, 26] | 10 [8, 13] | 10 [8, 13] | 14 [13, 15] | 10 [8, 13] |
| Time to first full enteral feeding, days | 74 [74, 74] | 32 [25, 45] | 32 [26, 44] | 55 [41, 80] | 28 [22, 45] |
| Duration of antibiotics for SIP, days | 7 [7, 7] | 11 [8, 16] | 12 [8, 16] | 23 [21, 24] | 9 [7, 17] |
†, ten SIP infants were lost to follow-up with unknown survival status, so they were not included in the calculation of case fatality rate. ‡, calculated among SIP survivors. SIP, spontaneous intestinal perforation; VPIs, very preterm infants; IQR, interquartile range.
Among 122 infants with surgical management for SIP, 84 (68.9%) infants received laparotomy only (Table 2). The remaining 38 (31.1%) infants received peritoneal drainage as the initial intervention, with only nine (7.4%) treated solely by peritoneal drainage, and 29 (23.8%) by secondary laparotomy following drainage. In total, there were 113 (92.6%) infants ultimately received laparotomy. Infants with lower GA were more likely to have peritoneal drainage only after SIP (P<0.01).
Eight surgical cases were transferred out and lost to follow-up. Among the remaining 114 surgical SIP infants, nineteen (16.7%) infants died, with sixteen (14.0%) SIP-related deaths (Table 2). Overall case fatality rates were similar between infants initially treated with laparotomy and with peritoneal drainage (16.9% vs. 16.2%; P=0.93). Surgical infants with complete care had lower overall and SIP-related case fatality rates.
Maternal and neonatal characteristics of SIP
Maternal and neonatal characteristics of infants with and without SIP are summarized in Table 3. Maternal characteristics of infants with and without SIP were similar, except for multiple pregnancy (40.7% vs. 30.4%; P<0.01) and diabetes (9.6% vs. 18.5%; P<0.01). Infants with SIP were of lower GA and birth weight, were more likely to be male (68.0% vs. 56.2%; P<0.01) and SGA (9.3% vs. 4.9%; P=0.01), and had a higher incidence of severe IVH (17.3% vs. 6.3%; P<0.01) compared to infants without SIP. A greater proportion of infants in the SIP group underwent umbilical artery catheterization and were exposed to steroids, antibiotics, nitric oxide, inotropes, invasive ventilation, and blood transfusion within the first 7 postnatal days. Caffeine was less frequently used in infants with SIP.
Table 3
| Characteristics | No SIP | SIP | Total | P value |
|---|---|---|---|---|
| Number of infants | 15,664 | 150 | 15,814 | |
| Maternal characteristics, n/N (%) | ||||
| Maternal age, years, mean (SD) | 31.09 (4.95) | 31.02 (5.02) | 31.09 (4.95) | 0.86 |
| Assisted reproductive technology | 2,647/15,664 (16.9) | 27/150 (18.0) | 2,674/15,814 (16.9) | 0.72 |
| Multiple pregnancy | 4,759/15,664 (30.4) | 61/150 (40.7) | 4,820/15,814 (30.5) | <0.01 |
| Diabetes | 2,867/15,528 (18.5) | 14/146 (9.6) | 2,881/15,674 (18.4) | <0.01 |
| Hypertension | 2,997/15,532 (19.3) | 25/148 (16.9) | 3,022/15,680 (19.3) | 0.46 |
| Chorioamnionitis | 2,361/13,016 (18.1) | 24/117 (20.5) | 2,385/13,133 (18.2) | 0.51 |
| Premature rupture of membranes | 8,674/14,895 (58.2) | 82/143 (57.3) | 8,756/15,038 (58.2) | 0.83 |
| Cesarean delivery | 8,938/15,621 (57.2) | 96/150 (64.0) | 9,034/15,771 (57.3) | 0.09 |
| Antenatal magnesium sulfate | 7,307/13,908 (52.5) | 65/118 (55.1) | 7,372/14,026 (52.6) | 0.58 |
| Antenatal steroids | 11,574/14,722 (78.6) | 100/131 (76.3) | 11,674/14,853 (78.6) | 0.53 |
| Antenatal antibiotics | 6,365/13,833 (46.0) | 49/115 (42.6) | 6,414/13,948 (46.0) | 0.47 |
| Neonatal characteristics, n/N (%) | ||||
| Gestational age, weeks, median [IQR] | 30 [29, 31] | 30 [28, 31] | 30 [29, 31] | 0.01 |
| Birth weight, grams, mean (SD) | 1,330.36 (318.13) | 1,213.19 (349.46) | 1,329.25 (318.63) | <0.01 |
| Male | 8,796/15,646 (56.2) | 102/150 (68.0) | 8,898/15,796 (56.3) | <0.01 |
| SGA | 774/15,646 (4.9) | 14/150 (9.3) | 788/15,796 (5.0) | 0.01 |
| Apgar score <7 at 1 min | 3,672/15,347 (23.9) | 46/150 (30.7) | 3,718/15,497 (24.0) | 0.05 |
| Apgar score <7 at 5 min | 1,043/14,887 (7.0) | 10/148 (6.8) | 1,053/15,035 (7.0) | 0.91 |
| Intensive resuscitation at delivery room | 606/15,132 (4.0) | 10/142 (7.0) | 616/15,274 (4.0) | 0.06 |
| Respiratory distress syndrome | 11,392/15,640 (72.8) | 112/149 (75.2) | 11,504/15,789 (72.9) | 0.52 |
| Severe IVH | 879/14,041 (6.3) | 22/127 (17.3) | 901/14,168 (6.4) | <0.01 |
| Early-onset sepsis | 204/15,664 (1.3) | 11/150 (7.3) | 215/15,814 (1.4) | 0.14 |
| Treatment in the first 7 days, n/N (%) | ||||
| Postnatal steroids | 220/15,664 (1.4) | 5/150 (3.3) | 225/15,814 (1.4) | 0.04 |
| Postnatal NSAIDs | 1,428/15,664 (9.1) | 17/150 (11.3) | 1,445/15,814 (9.1) | 0.35 |
| Antibiotics | 13,952/15,664 (89.1) | 144/150 (96.0) | 14,096/15,814 (89.1) | 0.01 |
| Surfactant | 8,552/15,664 (54.6) | 73/150 (48.7) | 8,625/15,814 (54.5) | 0.15 |
| Caffeine | 12,203/15,664 (77.9) | 101/150 (67.3) | 12,304/15,814 (77.8) | <0.01 |
| Nitric oxide | 130/15,664 (0.8) | 5/150 (3.3) | 135/15,814 (0.9) | <0.01 |
| Inotropes | 3,271/15,664 (20.9) | 68/150 (45.3) | 3,339/15,814 (21.1) | <0.01 |
| Umbilical artery catheters | 759/15,664 (4.8) | 14/150 (9.3) | 773/15,814 (4.9) | 0.01 |
| Umbilical vein catheters | 6,145/15,664 (39.2) | 56/150 (37.3) | 6,201/15,814 (39.2) | 0.63 |
| Invasive ventilation | 6,168/15,664 (39.4) | 128/150 (85.3) | 6,296/15,814 (39.8) | <0.01 |
| Transfusion | 3,162/15,664 (20.2) | 81/150 (54.0) | 3,243/15,814 (20.5) | <0.01 |
VPI, very preterm infant; SIP, spontaneous intestinal perforation; SD, standard deviation; IQR, interquartile range; SGA, small for gestational age; IVH, intraventricular hemorrhage; NSAID, nonsteroidal anti-inflammatory drug.
Outcomes of SIP infants
As shown in Table 4, 29.3% (41/140) of infants with SIP survived without major morbidities, significantly lower than those without SIP (9,279/15,664, 59.2%) (P<0.01). Univariate comparisons revealed the rates of overall death, LOS, and BPD were higher in the SIP group, compared with the non-SIP group. Growth velocity during hospitalization was lower in the SIP survivors, and the incidence of EUGR was higher regardless of which diagnostic criteria were used. SIP infants were also more likely to receive central catheters and invasive ventilation, more transfusion, and longer duration of parenteral nutrition and hospital stay.
Table 4
| Outcomes | No SIP | SIP | Total | P value |
|---|---|---|---|---|
| Number of infants | 15,664 | 150 | 15,814 | |
| Neonatal outcomes, n/N (%) | ||||
| Survival without major morbidities | 9,279/15,664 (59.2) | 41/140 (29.3) | 9,320/15,804 (59.0) | <0.01 |
| Overall death | 1,667/15,664 (10.6) | 41/140 (29.3) | 1,708/15,804 (10.8) | <0.01 |
| Major morbidities† | ||||
| LOS | 871/13,997 (6.2) | 17/99 (17.2) | 888/14,096 (6.3) | <0.01 |
| BPD | 3,730/13,997 (26.6) | 48/99 (48.5) | 3,778/14,096 (26.8) | <0.01 |
| PVL | 604/13,091 (4.6) | 8/93 (8.6) | 612/13,184 (4.6) | 0.06 |
| Severe ROP | 389/12,299 (3.2) | 3/90 (3.3) | 392/12,389 (3.2) | 0.92 |
| Growth assessment at discharge† | ||||
| Growth velocity, g/(kg·d), median (IQR) | 10.16 (8.40, 11.70) | 9.40 (8.03, 10.96) | 10.16 (8.40, 11.70) | <0.01 |
| EUGR, n/N (%) | ||||
| Weight < P10 | 6,163/13,240 (46.5) | 65/98 (66.3) | 62,28/13,338 (46.7) | <0.01 |
| Decrease in weight z score >2 from birth to discharge | 2,426/13,240 (18.3) | 44/98 (44.9) | 24,70/13,338 (18.5) | <0.01 |
| Treatment during hospitalization† | ||||
| Central venous catheters, n/N (%) | 10,934/15,664 (69.8) | 112/140 (80.0) | 11,046/15,804 (69.9) | <0.01 |
| Duration, days, median [IQR] | 21 [12, 31] | 26 [10, 39] | 21 [12, 32] | 0.06 |
| Invasive ventilation, n/N (%) | 6,755/15,664 (43.1) | 134/140 (95.7) | 6,889/15,804 (43.6) | <0.01 |
| Duration, days, median [IQR] | 4 [2, 9] | 7 [3, 15] | 4 [2, 10] | <0.01 |
| Duration of parenteral nutrition, days, median [IQR] | 21 [13, 32] | 39 [28, 50] | 21 [13, 32] | <0.01 |
| Times of transfusion, median [IQR] | 1 [0, 2] | 3 [2, 6] | 1 [0, 2] | <0.01 |
| Length of NICU stay, days, median [IQR] | 46 [34, 60] | 56 [42, 79] | 46 [34, 60] | <0.01 |
†, rates of major morbidities, growth assessment at discharge, times of transfusion, length of NICU stay, and duration of central venous catheter, invasive ventilation, and parenteral nutrition during hospitalization were calculated among SIP survivors. VPI, very preterm infant; SIP, spontaneous intestinal perforation; LOS, late-onset sepsis; BPD, bronchopulmonary dysplasia; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; EUGR, extrauterine growth restriction; IQR, interquartile range; NICU, neonatal intensive care unit.
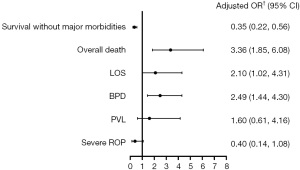
Multivariate analysis showed the odds of survival without major morbidities among infants with SIP were significantly lower than those without SIP after adjustment [adjusted odds ratio (OR) 0.35; 95% confidence interval (CI): 0.22 to 0.56] (Figure 2). SIP was associated with a higher risk of overall death (adjusted OR 3.36; 95% CI: 1.85 to 6.08), LOS (adjusted OR 2.10; 95% CI: 1.02 to 4.31), and BPD (adjusted OR 2.49; 95% CI: 1.44 to 4.30).
Discussion
This was the first large multicenter cohort study providing detailed epidemiology of SIP among VPIs in Chinese NICUs. The incidence of SIP was 1.0% among VPIs, with an increasing trend among infants at lower GA. More than one fourth of SIP infants died, and only 29.3% survived without any major morbidities. SIP was independently associated with twofold to threefold higher odds of overall death, LOS, and BPD. Peritoneal drainage was provided to one third of surgical VPIs with SIP, while over 90% of infants ultimately received laparotomy.
SIP has become a common surgical bowel disease in preterm infants. It was easily confounded by NEC or gastric perforation but was unlikely to be misclassified in our study as most cases were confirmed by laparotomy. Our findings of higher incidence of SIP in infants with lower GA were consistent with previous studies, but the overall incidence of SIP among VPIs was slightly lower than reported (5,27). Elgendy et al. showed a 1.6% incidence of SIP in infants with BW <1,500 g and with GA ≤32 weeks using a national dataset of the United States, and 89.9% of SIP cases were in the category of GA ≤28 weeks (5). Infants included in our study were mostly born at 28+0–31+6 weeks’ gestation, which might account for the comparatively lower incidence of SIP. Only 0.8% of infants with GA 28+0–29+6 weeks developed SIP, while the incidence was as high as 2.4% in infants with GA 24+0–25+6 weeks. However, the incidence of SIP did not further decrease in the larger GA group, probably due to the complicated and critical condition of infants admitted to tertiary NICUs in CHNN. Despite the relatively lower risk of SIP in infants with GA ≥28 weeks, attention should also be paid to the prevention of SIP among this group of infants, as they comprised a major proportion of VPIs and the absolute number of SIP cases was quite large. More studies are needed to identify risk factors of SIP, which may help establish specific prevention strategies. Interestingly, we found caffeine administration was more common in infants without SIP, indicating that it might play a protective role in the development of SIP. Vongbhavit et al. reported similar results in a case-control study of preterm infants with birth weight <2,000 g and GA <34 weeks (28), but the causal relationship between caffeine and SIP remained to be verified.
A number of studies have demonstrated that infants with SIP have higher odds of death or major morbidities (4,5,9-11), and our results showed similar findings. More than a quarter of VPIs with SIP died, most of which were SIP-related and occurred within two weeks after the onset of SIP. Overall death rate of SIP was reported to be 19.2–24.7% among VPIs in previous large population-based studies (4,5), slightly lower than our results. It might be partly explained by the selection bias of the cohort in this study. All participating hospitals in CHNN were tertiary centers and the infants involved were usually much sicker than those from lower-level healthcare facilities, leading to overestimation of the case fatality rates of SIP. Moreover, quite a few infants with SIP were discharged against medical advice and the case fatality rates of SIP were higher in those without complete care. Encouraging parents to have their babies receive complete care for SIP might reduce the mortality of VPIs. For SIP survivors, the risk of LOS and BPD was also doubled, independent of GA and other confounding factors. Multiple mechanisms, such as severe inflammatory response, compromised nutrition, and prolonged ventilation after SIP, are expected to be responsible for increased risk of the morbidities but further studies are needed. The strong association between SIP and poor prognosis emphasized the importance of preventing SIP among VPIs.
Almost all survived SIP infants required surgical management. In our study, only eight infants with SIP were managed conservatively without any surgical intervention, of which four died without a chance of surgery. The other four who survived were relatively stable and recovered after less invasive options, such as peritoneal needle aspiration. Among VPIs with SIP who received surgery, more than half received laparotomy only and about three quarters of infants initially treated with peritoneal drainage received secondary laparotomy, indicating that laparotomy was the most commonly used surgical intervention for SIP in China. In the literature, however, peritoneal drainage was performed in the majority (64.3–89.3%) of SIP infants with a varied proportion (24.1–68.8%) of infants subsequently requiring laparotomy (29-34). Considering that peritoneal drainage is allowed to be performed at the bedside and avoids surgical complications, it is generally conducted as an alternative treatment for SIP, temporarily or definitively, especially in infants who are too unstable to tolerate laparotomy. The infants enrolled in previous studies were less mature (usually ELBW infants) than those in our study, which might contribute to the relatively higher rate of peritoneal drainage as the initial procedure for SIP. The optimal surgical treatment of SIP is still controversial. Both randomized controlled trials (35-37) and observational studies (29,32,38,39) have demonstrated the type of initial operation has no impact on survival among preterm infants with intestinal perforation, similar to our findings. However, most studies mentioned above did not distinguish SIP from NEC and did not separately report the outcomes for SIP. It is noteworthy that infants with peritoneal drainage only had a significantly higher case fatality rate compared with those receiving secondary laparotomy after drainage in our study, whereas other studies reported similar survival rates between the two groups (30,31). The reason for these infants to receive peritoneal drainage only was probably that they were critically ill and incapable of tolerating laparotomy, rather than that peritoneal drainage made laparotomy unnecessary. Analysis of the characteristics of infants who “successfully” avoided laparotomy after drainage was limited due to a small sample size (only four cases) and larger-scale studies are needed.
In the postoperative recovery of SIP, parenteral nutrition support plays a crucial role. Enteral feeding was not started until at least one week after SIP in all GA groups in our study. Previous studies have reported time to initiation of enteral nutrition in SIP infants ranging from 6 to 26 days (12,31,33,40,41). Parenteral nutrition is therefore essential and prolonged for SIP survivors, especially for infants born at lower GA (12,31,41). Our results showed the median time to achieve first full enteral feeding was 54 (IQR 33–102) days in infants at 24+0–25+6 weeks’ gestation, almost twice that of infants at 30+0–31+6 weeks’ gestation. However, Eicher et al. documented a significantly shorter time to full enteral feeding after SIP [15 (IQR 12–19) days] among ELBW infants in a single-center retrospective study (40), suggesting the potential for nutrition strategy optimization for infants with SIP.
The study had several limitations. First, all enrolled infants came from tertiary NICUs and data from less developed regions were lacking, which might restrict the generalizability of our findings. Second, details of surgical management for SIP were not collected, such as the reason for peritoneal drainage or laparotomy, the selection of specific procedures (enterostomy or primary anastomosis), and the criteria to determine the need for secondary laparotomy after peritoneal drainage. These information would further help to evaluate the benefits and risks of different interventions. Third, we did not follow up neurodevelopmental and long-term outcomes in survivors, which are also important for a complete assessment of SIP. Fourth, many antenatal and postnatal factors have been reported to be associated with SIP, such as magnesium sulfate, indomethacin, and hydrocortisone (16), but we did not evaluate medication use and association with SIP in our population.
Conclusions
Around 1% of VPIs in Chinese NICUs developed SIP, associated with an alarmingly high risk of mortality and morbidities. The majority of infants with SIP underwent laparotomy as initial or subsequent surgical treatment. More studies are needed on effective prevention interventions and evidence-based surgical management strategies for SIP.
Acknowledgments
We thank the data abstractors from the Chinese Neonatal Network. We thank all the staff at the Chinese Neonatal Network coordinating center for providing organizational support (Yulan Lu, PhD; Tongling Yang, RN; Jie Yang, PhD; Hao Yuan, RN; Li Wang, RN; Lin Yuan, PhD).
Group information of the Chinese Neonatal Network: Chairmen: Shoo K. Lee, MBBS, Mount Sinai Hospital, University of Toronto; Chao Chen, MD, Children’s Hospital of Fudan University. Vice-Chairmen: Lizhong Du, MD, Children’s Hospital of Zhejiang University School of Medicine; Wenhao Zhou, Children’s Hospital of Fudan University.
Site principle investigators of the Chinese Neonatal Network: Children’s Hospital of Fudan University: Yun Cao, MD; The Third Affiliated Hospital of Zhengzhou University: Xiuyong Chen, MD; Guangzhou Women and Children’s Medical Center: Huyan Zhang, MD; Tianjin Obstetrics & Gynecology Hospital: Xiuying Tian, MD; Gansu Provincial Maternity and Child Care Hospital: Jingyun Shi, MD; Northwest Women’s and Children’s Hospital: Zhankui Li, MD; Shenzhen Maternity and Child Health Care Hospital: Chuanzhong Yang, MD; Guizhou Women and Children’s Hospital: Ling Liu, MD; Suzhou Municipal Hospital Affiliated to Nanjing Medical University: Zuming Yang, MD; Shengjing Hospital of China Medical University: Jianhua Fu, MD; Children’s Hospital of Shanxi: Yong Ji, MD; Quanzhou Women and Children’s Hospital: Dongmei Chen, MD; Fujian Women and Children’s Medical Center: Changyi Yang, MD; Children’s Hospital of Nanjing Medical University: Rui Chen, MD; Hunan Children’s Hospital: Xiaoming Peng, MD; Qingdao Women and Children’s Hospital: Ruobing Shan, MD; Nanjing Maternity and Child Health Care Hospital: Shuping Han, MD; The First Bethune Hospital of Jilin University: Hui Wu, MD; The First Affiliated Hospital of Anhui Medical University: Lili Wang, MD; Women and Children’s Hospital of Guangxi Zhuang Autonomous Region: Qiufen Wei, MD; The First Affiliated Hospital of Xinjiang Medical University: Mingxia Li, MD; Foshan Women and Children’s Hospital: Yiheng Dai, MD; The Affiliated Hospital of Qingdao University: Hong Jiang, MD; Henan Children’s Hospital: Wenqing Kang, MD; Children’s Hospital of Shanghai: Xiaohui Gong, MD; Chongqing Health Care Center for Women and Children: Xiaoyun Zhong, MD; Children’s Hospital of Chongqing Medical University: Yuan Shi, MD; Wuxi Maternity and Child Healthcare Hospital: Shanyu Jiang, MD; Children’s Hospital of Soochow University: Bing Sun, MD; People’s Hospital of Xinjiang Uygur Autonomous Region: Long Li, MD; Yuying Children’s Hospital Affiliated to Wenzhou Medical University: Zhenlang Lin, MD; Shanghai First Maternity and Infant Hospital: Jiangqin Liu, MD; Anhui Provincial Hospital: Jiahua Pan, MD; Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine: Hongping Xia, MD; Qilu Children’s Hospital of Shandong University: Xiaoying Li, MD; The First Affiliated Hospital of Zhengzhou University: Falin Xu, MD; General Hospital of Ningxia Medical University: Yinping Qiu, MD; Hebei Children’s Hospital: Li Ma, MD; Hainan Women and Children’s Hospital: Ling Yang, MD; The Second Xiangya hospital of Central South University: Xiaori He, MD; Ningbo Women & Children Hospital: Yanhong Li, MD; Xiamen Children’s Hospital: Deyi Zhuang, MD; Shaanxi Provincial People’s Hospital: Qin Zhang, MD; The Affiliated Hospital of Southwest Medical University: Wenbin Dong, MD; Shanghai Children’s Medical Center Affiliated to Shanghai Jiaotong University School of Medicine: Jianhua Sun, MD; First Affiliated Hospital of Kunming Medical University: Kun Liang, MD; Changzhou Maternal and Children Health Care Hospital: Huaiyan Wang, MD; Shenzhen Children’s Hospital: Jinxing Feng, MD; Jiangxi Provincial Children’s Hospital: Liping Chen, MD; Xiamen Maternity and Child Health Care Hospital: Xinzhu Lin, MD; Zhuhai Center for Maternal and Child Health Care: Chunming Jiang, MD; Guangdong Women and Children’s Hospital: Chuan Nie, MD; Wuhan Children’s Hospital: Lingkong Zeng, MD; Beijing Children’s Hospital of Capital Medical University: Mingyan Hei, MD; Maternal and Children Hospital of Shaoxing: Hongdan Zhu, MD; The First People’s Hospital of Yunnan Province: Hongying Mi, MD; Dehong People’s Hospital of Yunnan Province: Zhaoqing Yin, MD; First Affiliated Hospital of Xi'an Jiaotong University: Hongxia Song, MD; Inner Mongolia Maternal and Child Health Care Hospital: Hongyun Wang, MD; Dalian Municipal Women and Children’s Medical Center: Dong Li, MD; Lianyungang Maternal and Children Health Hospital: Yan Gao, MD; Children’s Hospital Affiliated to Capital Institute of Pediatrics: Yajuan Wang, MD; Anhui Children’s Hoospital: Liying Dai, MD; Fuzhou Children’s Hospital of Fujian Province: Liyan Zhang, MD; Kunming Children’s Hospital: Yangfang Li, MD; Shenzhen Hospital of Hongkong University: Qianshen Zhang, MD; Peking Union Medical College Hospital: Guofang Ding, MD; Obstetrics & Gynecology Hospital of Fudan University: Jimei Wang, MD; The Affiliated Hospital of Guizhou Medical University: Xiaoxia Chen, MD; Qinghai Women and Children Hospital: Zhen Wang, MD; The International Peace Maternity & Child Health Hospital of China Welfare Institute: Zheng Tang, MD; Children’s Hospital of Zhejiang University: Xiaolu Ma, MD; Inner Mongolia People’s Hospital: Xiaomei Zhang, MD; Xiamen Humanity Hospital: Xiaolan Zhang, MD; Shanghai General Hospital: Fang Wu, MD; The First People’s Hospital of Yinchuan: Yanxiang Chen, MD; The Third Hospital of Nanchang: Ying Wu, MD; Advisor: Joseph Ting, MBBS, University of Alberta.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-584/rc
Data Sharing Statement: https://tp.amegroups.com/article/view/10.21037/tp-23-584/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-584/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-584/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Board of Children’s Hospital of Fudan University (No. #CHFU 2018-296) and recognized by all participating hospitals. A waiver of informed consent was granted owing to the use of deidentified patient data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pumberger W, Mayr M, Kohlhauser C, et al. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg 2002;195:796-803. [Crossref] [PubMed]
- Kubota A, Yamanaka H, Okuyama H, et al. Focal intestinal perforation in extremely-low-birth-weight neonates: etiological consideration from histological findings. Pediatr Surg Int 2007;23:997-1000. [Crossref] [PubMed]
- Fisher JG, Jones BA, Gutierrez IM, et al. Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J Pediatr Surg 2014;49:1215-9. [Crossref] [PubMed]
- Shah J, Singhal N, da Silva O, et al. Intestinal perforation in very preterm neonates: risk factors and outcomes. J Perinatol 2015;35:595-600. [Crossref] [PubMed]
- Elgendy MM, Othman HF, Heis F, et al. Spontaneous intestinal perforation in premature infants: a national study. J Perinatol 2021;41:1122-8. [Crossref] [PubMed]
- Meyer CL, Payne NR, Roback SA. Spontaneous, isolated intestinal perforations in neonates with birth weight less than 1,000 g not associated with necrotizing enterocolitis. J Pediatr Surg 1991;26:714-7. [Crossref] [PubMed]
- Attridge JT, Herman AC, Gurka MJ, et al. Discharge outcomes of extremely low birth weight infants with spontaneous intestinal perforations. J Perinatol 2006;26:49-54. [Crossref] [PubMed]
- Swanson JR, Hair A, Clark RH, et al. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J Perinatol 2022;42:423-9. [Crossref] [PubMed]
- Zozaya C, Shah J, Pierro A, et al. Neurodevelopmental and growth outcomes of extremely preterm infants with necrotizing enterocolitis or spontaneous intestinal perforation. J Pediatr Surg 2021;56:309-16. [Crossref] [PubMed]
- Shah TA, Meinzen-Derr J, Gratton T, et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol 2012;32:552-8. [Crossref] [PubMed]
- Wadhawan R, Oh W, Hintz SR, et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol 2014;34:64-70. [Crossref] [PubMed]
- Wadhawan R, Oh W, Vohr BR, et al. Spontaneous intestinal perforation in extremely low birth weight infants: association with indometacin therapy and effects on neurodevelopmental outcomes at 18-22 months corrected age. Arch Dis Child Fetal Neonatal Ed 2013;98:F127-32. [Crossref] [PubMed]
- Adant I, Miserez M, Naulaers G, et al. Long-term outcomes of very low birth weight infants with spontaneous intestinal perforation: A retrospective case-matched cohort study. J Pediatr Surg 2019;54:2084-91. [Crossref] [PubMed]
- Karila K, Anttila A, Iber T, et al. Outcomes of surgery for necrotizing enterocolitis and spontaneous intestinal perforation in Finland during 1986-2014. J Pediatr Surg 2018;53:1928-32. [Crossref] [PubMed]
- Vaidya R, Yi JX, O'Shea TM, et al. Long-Term Outcome of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation. Pediatrics 2022;150:e2022056445. [Crossref] [PubMed]
- Thakkar PV, Sutton KF, Detwiler CB, et al. Risk factors and epidemiology of spontaneous intestinal perforation among infants born at 22-24 weeks’ gestational age. J Perinatol 2024;44:94-9. [Crossref] [PubMed]
- Cao Y, Jiang S, Sun J, et al. Assessment of Neonatal Intensive Care Unit Practices, Morbidity, and Mortality Among Very Preterm Infants in China. JAMA Netw Open 2021;4:e2118904. [Crossref] [PubMed]
- Hei M, Li X, Shi Y, et al. Chinese Neonatal Network: a national protocol for collaborative research and quality improvement in neonatal care. BMJ Open 2022;12:e051175. [Crossref] [PubMed]
- Sun J, Cao Y, Hei M, et al. Data Quality Improvement and Internal Data Audit of the Chinese Neonatal Network Data Collection System. Front Pediatr 2021;9:711200. [Crossref] [PubMed]
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179-201. [Crossref] [PubMed]
- Jiang S, Huang X, Zhang L, et al. Estimated Survival and Major Comorbidities of Very Preterm Infants Discharged Against Medical Advice vs Treated With Intensive Care in China. JAMA Netw Open 2021;4:e2113197. [Crossref] [PubMed]
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59. [Crossref] [PubMed]
- Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529-34. [Crossref] [PubMed]
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723-9. [Crossref] [PubMed]
- The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991-9. [Crossref] [PubMed]
- WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76-85. [PubMed]
- Suply E, Leclair MD, Neunlist M, et al. Spontaneous Intestinal Perforation and Necrotizing Enterocolitis: A 16-Year Retrospective Study from a Single Center. Eur J Pediatr Surg 2015;25:520-5. [Crossref] [PubMed]
- Vongbhavit K, Underwood MA. Intestinal perforation in the premature infant. J Neonatal Perinatal Med 2017;10:281-9. [Crossref] [PubMed]
- Ahle S, Badru F, Damle R, et al. Multicenter retrospective comparison of spontaneous intestinal perforation outcomes between primary peritoneal drain and primary laparotomy. J Pediatr Surg 2020;55:1270-5. [Crossref] [PubMed]
- Emil S, Davis K, Ahmad I, et al. Factors associated with definitive peritoneal drainage for spontaneous intestinal perforation in extremely low birth weight neonates. Eur J Pediatr Surg 2008;18:80-5. [Crossref] [PubMed]
- Jakaitis BM, Bhatia AM. Definitive peritoneal drainage in the extremely low birth weight infant with spontaneous intestinal perforation: predictors and hospital outcomes. J Perinatol 2015;35:607-11. [Crossref] [PubMed]
- Mishra P, Foley D, Purdie G, et al. Intestinal perforation in premature neonates: The need for subsequent laparotomy after placement of peritoneal drains. J Paediatr Child Health 2016;52:272-7. [Crossref] [PubMed]
- Chiu B, Pillai SB, Almond PS, et al. To drain or not to drain: a single institution experience with neonatal intestinal perforation. J Perinat Med 2006;34:338-41. [Crossref] [PubMed]
- Fatemizadeh R, Mandal S, Gollins L, et al. Incidence of spontaneous intestinal perforations exceeds necrotizing enterocolitis in extremely low birth weight infants fed an exclusive human milk-based diet: A single center experience. J Pediatr Surg 2021;56:1051-6. [Crossref] [PubMed]
- Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med 2006;354:2225-34. [Crossref] [PubMed]
- Rees CM, Eaton S, Kiely EM, et al. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg 2008;248:44-51. [Crossref] [PubMed]
- Blakely ML, Tyson JE, Lally KP, et al. Initial Laparotomy Versus Peritoneal Drainage in Extremely Low Birthweight Infants With Surgical Necrotizing Enterocolitis or Isolated Intestinal Perforation: A Multicenter Randomized Clinical Trial. Ann Surg 2021;274:e370-80. [Crossref] [PubMed]
- Blakely ML, Tyson JE, Lally KP, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics 2006;117:e680-7. [Crossref] [PubMed]
- Rakshasbhuvankar A, Rao S, Minutillo C, et al. Peritoneal drainage versus laparotomy for perforated necrotising enterocolitis or spontaneous intestinal perforation: a retrospective cohort study. J Paediatr Child Health 2012;48:228-34. [Crossref] [PubMed]
- Eicher C, Seitz G, Bevot A, et al. Surgical management of extremely low birth weight infants with neonatal bowel perforation: a single-center experience and a review of the literature. Neonatology 2012;101:285-92. [Crossref] [PubMed]
- Cass DL, Brandt ML, Patel DL, et al. Peritoneal drainage as definitive treatment for neonates with isolated intestinal perforation. J Pediatr Surg 2000;35:1531-6. [Crossref] [PubMed]


